Polymer nanovectors to improve efficacy of photodynamic therapy (PDT): applications to antitumor strategy and regenerative medicine
Abstract
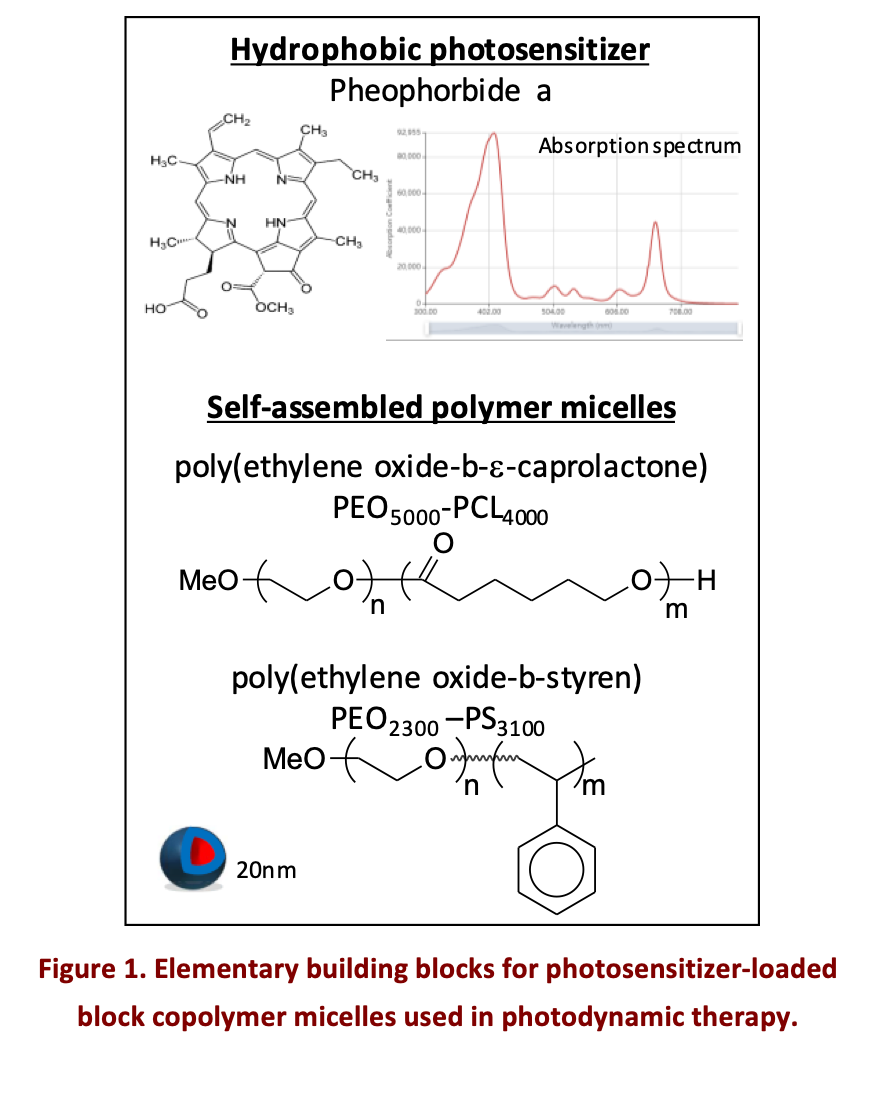
Photodynamic therapy (PDT), used clinically for dermatological, ophthalmological and oncological applications, is a photochemical therapeutic modality using photoexcitable agents named photosensitizers and light to locally generate reactive oxygen species (ROS) [1]. The majority of photosensitizers tend to form aggregates in aqueous media due to their low solubility, which greatly reduces their ROS production efficiency. The main strategy to overcome this problem is to encapsulate the photosensitizers within nanovectors, in particular polymer vectors, manufactured from biocompatible and biodegradable polymers that self-assemble to form nanovectors [2]. For more than 10 years, we have been evaluating different nanovectors based on amphiphilic block copolymers, with various compositions such as (poly(ethylene oxide)-block-poly(ε-caprolactone) or poly(ethylene oxide)-bloc-poly(styrene) or different morphologies (micelles, polymersomes or worm-like systems) [3–5] (Fig. 1). In most cases, we used Pheophorbide a as photosensitizer.
In antitumor strategy perspective, the photocytotoxicity of all systems was evaluated in various conditions, spanning from 2D cell lines such as HCT-116, or normal and tumor spheroids or in vivo. Our main results concerning antitumor strategy based on PDT is that 1) encapsulation always led to better cytotoxic PDT result; 2) 3D biological tests should be favored when possible; 3) crosslinking or mixtures of nanovectors may lead to PDT improvement; 4) THz spectroscopy is a powerful tool that revealed the extremely rapid kinetics of membrane permeability during illumination. Interestingly, we have more recently proposed working in sub-lethal PDT condition (meaning without affecting cell viability) in order to modify the behaviour of cells and tissues with a view to regenerative medicine. Indeed, oxidative stress, below a certain threshold, is known to stimulate a number of biological processes, such as wound healing and remodelling of the extracellular matrix. Thus, we propose to use calibrated-PDT (non-cytotoxic PDT) as a ROS-modulating technology to locally tune extracellular matrix in a calibrated manner to treat fibrotic condition for example.
References
- Á. Juarranz, Y. Gilaberte, S. González Cancers 2020, 12.
- M. Demazeau, L. Gibot, A.-F. Mingotaud, P. Vicendo, C. Roux, B. Lonetti Beilstein J. Nanotechnol. 2020, 11, 180.L. Gibot, A. Lemelle, U. Till, B. Moukarzel, A.-F. Mingotaud, V. Pimienta, P. Saint-Aguet, M.-P. Rols, M. Gaucher, F. Violleau et al. Biomacromolecules 2014, 15, 1443.
- Zheng, X.; Lordon, B.; Mingotaud, A.-F.; Vicendo, P.; Brival, R.; Fourquaux, I.; Gibot, L.; Gallot, G. Terahertz Adv. Sci. 2023, 10, 2300589.
- R. Brival, N. Ghafari, A.-F. Mingotaud, I. Fourquaux, V. Gilard, F. Collin, P. Vicendo, S. Balayssac, L. Gibot Int. J. Pharm. 2024, 663, 124589.
Acknowledgments
Authors acknowledge financial support from ANR TeraCellATR (ANR-21-CE42-0018), ANR LUMOS (ANR-23-CE18-0015), CNRS through the MITI interdisciplinary programs, Canceropole Grand Sud Ouest, and, more broadly, the various agencies that have been supporting our researches for several years.