Self-healing hydrogels
The present article refers to literature reports published from 2011 to 2019.
What are hydrogels?
Hydrogels are soft hydrophilic polymer tridimensional networks capable of absorbing high amounts of water (Figure 1) [1,2]. They exhibit mechanical and rheological properties typical of solid rubber-like materials. Hydrogels can be synthesized from both natural and synthetic polymers by either chemical or physical crosslinking. In chemical hydrogels, crosslinking results from the formation of the covalent bonds between the crosslinker and the macromolecular structure of the hydrogel matrix, while in physical hydrogels crosslinking is due to reversible physical interactions. High water content, softness and low surface friction give hydrogels a huge similarity to living tissues, opening up many opportunities for biomedical applications [2]. Historically, one of the major commercial applications of hydrogels was in the biomedical field for the production of hydrophilic soft contact lenses, which was reported as far back as 1959 [3]. Nowadays, hydrogels find relevant applications as scaffolds for tissue engineering [4], due to their ability to mimic the viscoelastic properties of the extracellular matrix, drug release formulations and as substrates for microfluidic and lab-on chip applications [5].
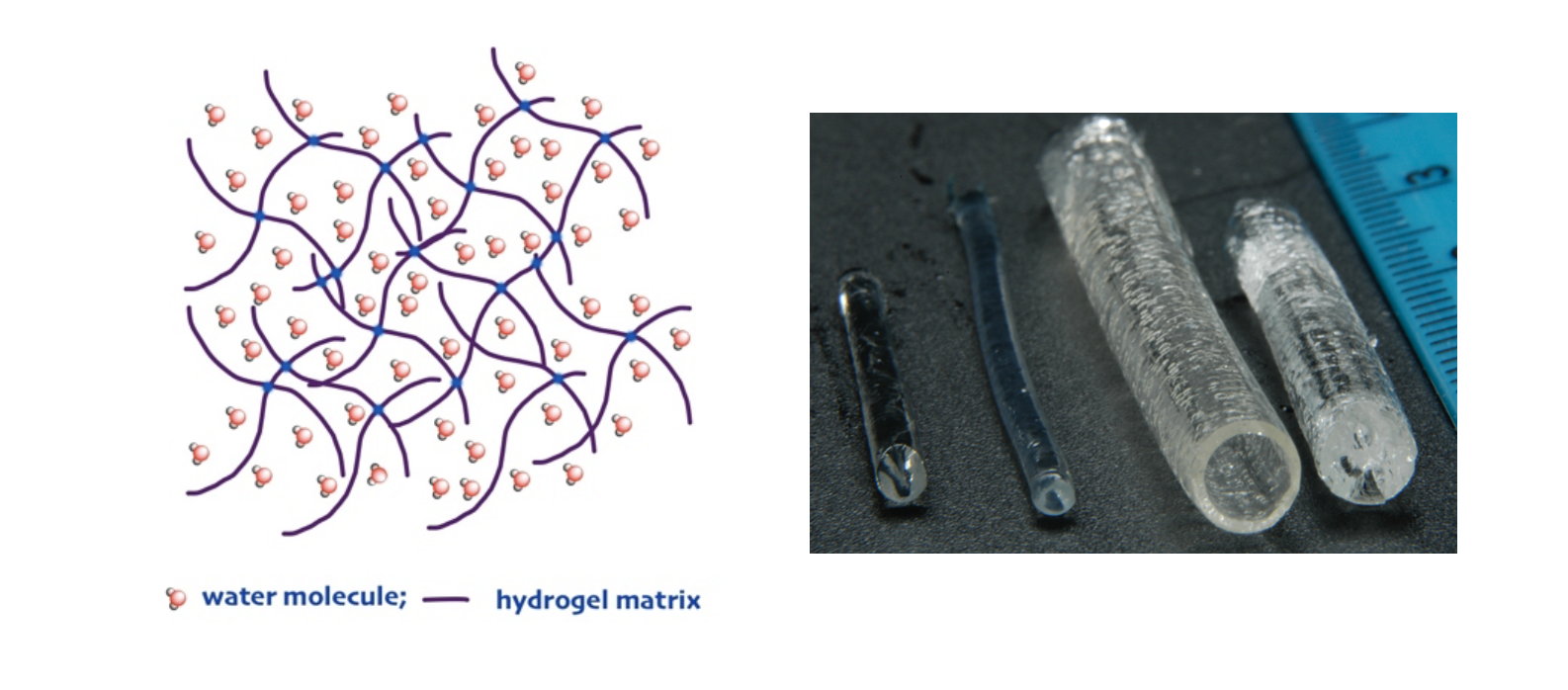
Figure 1. a) Structure of a tridimensional hydrogel network. b) Hydrogel samples with different shape and size.
What does self-healing hydrogel mean?
Self-healing hydrogels (SHHs) have the ability to repair structural damages and recover the original morphology and functions by spontaneously forming new bonds after the old ones have been degraded due to the intervention of external forces. [6,7]. The healing ability of SHHs can normally be exploited multiple times. In biomedical applications, this allows them to mimic the self-healing properties of native tissues [8]. The healing ability of SHHs derives from dynamic, that is, reversible cross-linking mechanisms and can therefore be activated cyclically. Reversibility arises from sensitiveness to external stimuli, which may include temperature, pH, and light changes. Thanks to the ability to adjust their physical and mechanical properties, SHHs can be used for a wide range of applications. SHHs can in fact be soft and strong materials, they can be cell adaptable, or they can exhibit shear thinning behavior, that is, their viscosity decreases under shear strain. They can be used in soft robotics, 3D printing, tissue engineering and drug delivery.
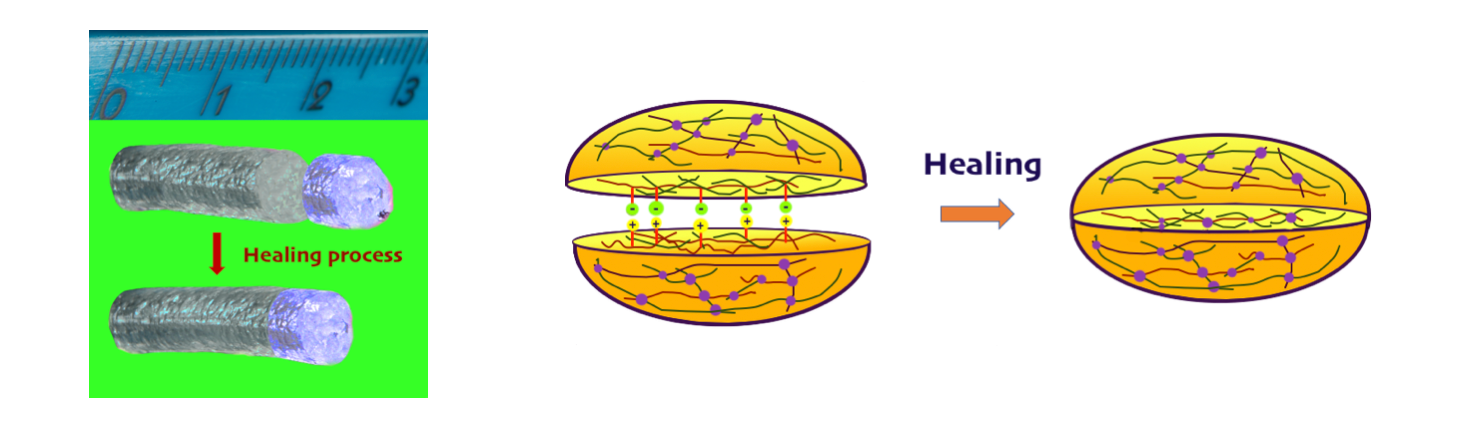
Figure 2. a) Self-healing hydrogel sample before and after damage reparation. b) Self-healing mechanism.
Self-healing Mechanisms
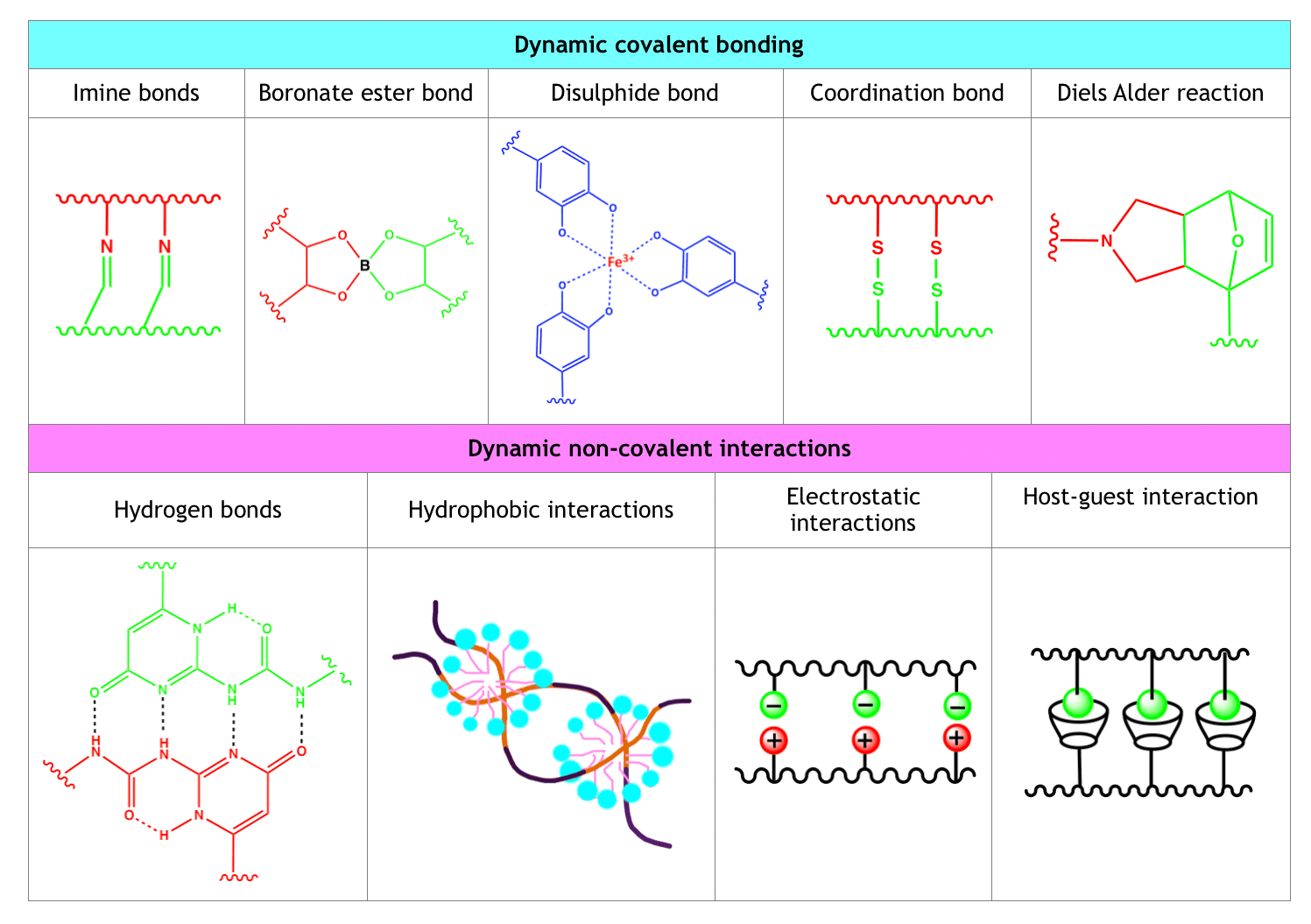
Hydrogels can self-heal following different mechanisms, that is, through the dynamic formation of covalent bonds, of non-covalent interactions, and through multi-mechanism interactions.
Table 1. Classification of Self-healing Mechanisms
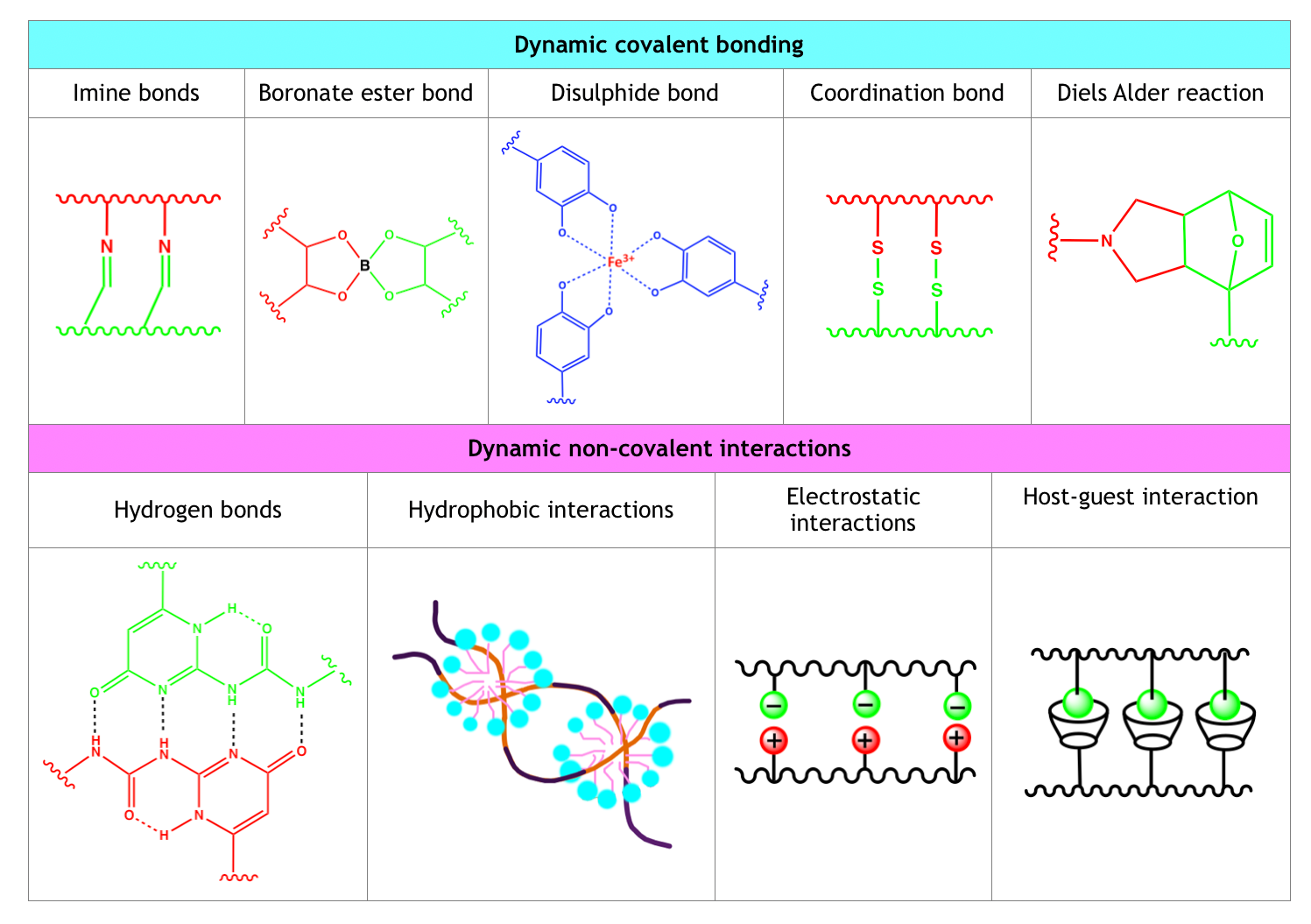
Dynamic Covalent Bonding
Dynamic covalent bonding mechanisms are characterized by the formation of strong inter-chain interactions and slower dynamic equilibria compared to non-covalent interaction-based self-healing mechanisms. Dynamic covalent bonding approaches are extremely versatile and, therefore, widely exploited.
Schiff base Formation
Schiff bases, or imines, are formed by the nucleophilic reaction between an amine and an aldehyde or a ketone.
The Schiff base formation reaction is a simple and convenient technique to produce SHHs. As an example, a SHH was obtained by reacting a dibenzaldehyde-terminated-polyethylene glycol (DF-PEG) with chitosan in mild conditions (20°C). The process was extremely fast (reaction time 60 s) and the hydrogel could be solubilized in acidic conditions and in the presence of enzymes [9].
Reversible Boronate Bond
The boronate ester group formation from boronic acid and diols has a pH- and glucose concentration-dependent stability. The reversibility of this reaction can be exploited to produce SHHs. Phenylboronic acid or phenylboronic acid-incorporated polymers were extensively used to prepare SHHs. For instance, mixing phenylboronic acid and diol-modified polyethylene glycol SHHs were obtained that exhibited pH-responsive tunable mechanical properties, and glucose-responsive size-dependent release of proteins [10]. An acid-sensitive SHH was also prepared by the reaction of a catechol-modified polymer and 1,3-benzenediboronic acid [11].
Catechol/Iron Coordinate Bond
SHHs can be formed by exploiting the pH-dependent catechol-iron coordination equilibrium (see also below the multiple interaction section). SHHs with high strength normally form at basic pHs. In one instance, a SHH was obtained by mixing catechol-modified polymers with iron oxide (Fe3O4) nanoparticles, favoring the reversible catechol-iron coordination occurring at the nanoparticle surface. These exhibited magnetic properties and solid-like mechanical properties superior to those obtained using soluble ion salts [12].
Disulfide Exchange
The disulfide exchange reaction is well known for being sensitive to redox potential and pH changes. The reversible dithiol/1,2-dithiolane exchange reaction is a convenient method for the synthesis of SHHs. This reaction undergoes rapid sol-gel transition shifting to neutral or weakly alkaline conditions [13].
Thermally reversible Diels-Alder reaction
Diels-Alder reactions normally require high temperature and a long reaction time and, therefore, are normally not suitable for the production of SHHs. However, they can be conveniently combined with other reversible interactions, such as electrostatic interaction [14], coordination bond [15] and imine bond to form SHH.
Non-covalent Interactions
Non-covalent interactions are notoriously less stable than covalent interactions, since they are more sensitive to different environmental conditions, including temperature and pH. Therefore, the crosslinked structures formed by this technique are normally more fragile.
Hydrogen Bonding
Reversible hydrogen bond formation can be used in different ways to obtain SHHs. Polyvinyl alcohol based SHHs were obtained using freezing/thawing procedures harnessing the ability of this polymer to form strong hydrogen bonds [16]. Recently, the cytosine- and guanosine-modified hyaluronic acid formed a SHH by Watson-Crick base pairing between the nucleobases precisely through hydrogen bonding [17]. The hydrogel exhibited pH-sensitive sol-gel transition, with a prevalence of sol state in the pH 6–8 range and of gel state at pH < 6 or > 8.
Hydrophobic Interactions
Hydrophobic interactions involve the aggregation of hydrophobes in aqueous media. An interesting example of the use of this approach for the synthesis of SHHs is provided by the micellar copolymerization of stearyl methacrylate with acrylamide carried out in an aqueous emulsion of sodium dodecyl sulfate [18]. In this process, the role of the surfactant micelles was to form physical crosslinks by interacting with the hydrophobic segments of the hydrophilic/hydrophobic copolymer.
Electrostatic Interactions
Another useful approach to the preparation of SHHs is the formation of polyelectrolyte-complexes between polyelectrolytes of opposite charge. In this case, the dynamic self-healing mechanism is related to the reversibility of the electrostatic interactions. A good example of this approach is the fabrication of SHHs from polyelectrolyte complexes between the anionically charged alginate and the positively charged 2-hydroxypropyltrimethyl ammonium chloride chitosan [19] The SHHs obtained were endowed with shear-thinning property, high adhesive behavior, and cytocompatibility.
Supramolecular Chemistry
In supramolecular chemistry, different chemical entities assemble via non-covalent interactions, such as van der Waals force, hydrogen bond, electrostatic interaction, and hydrophobic interaction. All these types of interactions can in principle be used to synthesize SHHs. The hydrogel healing process is in these instances normally triggered by external stimuli, including changes in temperature [20], UV-VIS irradiation [21], pH [22], and redox potentials [23]. However, in some instances self-healing takes place spontaneously, as in the case of the case of the host–guest interactions of β-cyclodextrin-modified hyaluronic acid, acting as host macromer, and adamantane-modified hyaluronic acid, acting as guest macromer [24].
Multi-mechanism Interactions
SHHs can be prepared also by multimodal mechanisms, as for instance either through host–guest interactions, or protein–ligand recognition, or hybrid non-covalent interactions and/or permanent/dynamic covalent bonding.
Hydrogen Bonding and Aromatic Interactions
A few examples are reported. A self-healing hydrogel was obtained by the self-assembly of an ABA tri-block copolymer where catechol-functionalized poly(N-isopropylacrylamide) and polyethylene oxide were the A and B blocks, respectively [25]. The self-healing mechanism was mediated by the hydrogen bonding and aromatic interactions of catechol. The SHH underwent thermally induced sol-gel transition and cyclically recovered its mechanical properties.
A pH responsive SHH was obtained by the one-step reaction of tannic acid, trivalent metal ions, and polyallylamine. Below pH 8, the SHH crosslinked mainly due to reversible hydrogen bonding, reaction/interactions between tannic acid and polyallylamine, and coordination between tannic acid and iron ions. At basic pH, irreversible bonds formed, deriving from the oxidation of the catechol moieties, which enhanced the modulus and hindered self-healing [26].
SHHs were also obtained from thiol-terminated polyethyleneglycol crosslinked with gold(I) ions [27].
Dual redox/pH-responsive SHHs were obtained by the acylhydrazone/disulfide bond dynamic covalent interaction [28]. The hydrogel displayed self-healing properties in acidic and basic conditions based on the acylhydrazone and disulfide bonds, respectively. Acylhydrazone bonds were sensitive to the catalytic action of aniline in neutral environment, while disulfide bonds were sensitive to redox variations.
Drug Delivery
β-Cyclodextrin-modified polyethyleneimine and adamantane-modified PEG gave rise to a host–guest SHH, which assembled with siRNA to form polyplexes [29]. The siRNA-encapsulated hydrogel showed good transfection efficiency, enhancing Cy5.5-siRNA uptake and maintaining the silencing of GFP when injected into the myocardium of GFP-expressing rats.
An injectable self-healing collagen-gold hydrogel for the localized and sustained release of photosensitive active principles was obtained exploiting the electrostatic interactions between the positively charged collagen and the negatively charged tetrachloroaurate ([AuCl4]−) ions [30].
A complex multi-modal chemotherapy/magnetic hyperthermia antitumor formulation was developed based on an injectable GC-DP hydrogel (see above) containing doxorubicin/docetaxel-loaded poly(lactic-co-glycolic acid) nanoparticles and iron oxide [31]. The system showed optimal in vivo antitumor efficacy under the action of alternating magnetic field. Ionic GC-DP hydrogels showed microwave-responsiveness and gave rise to the phenomenon of hyperthermia that could be exploited for tumor ablation [32].
Tissue Engineering
A SHH, obtained by the host–guest interaction of β-cyclodextrin-modified hyaluronic acid and adamantane with embedded endothelial progenitor cells was injected into the ischemic myocardium of rodents [33]. A remarkable vasculogenesis increase was observed compared to the treatment with plain EPCs.
SHHs were designed as injectable carriers for growth factors using PEG end-functionalized with four-fold hydrogen-bonding ureidopyrimidinone (UPy) moieties. UPy-modified PEG hydrogel incorporated with antifibrotic growth factor was delivered in a pocket introduced under the kidney capsule of rats [34]. These soluble precursors of these pH-switchable hydrogels could be injected through the long and narrow lumen of the catheter mapping system, and rapidly formed a hydrogel in contact with tissue.
3D printing
The SHHs based on the host–guest interaction of β-cyclodextrin- and adamantane-modified hyaluronic acid were employed for the 3D printing of high-resolution structures through printing of shearing-thinning ink hydrogel into self-healing support hydrogel [35]. The multicellular structures could be patterned, by printing of mesenchymal stem cells within an ink hydrogel into a support hydrogel containing 3T3 fibroblasts. The ink hydrogel was a physical host–guest SHH with shear thinning properties. It was injected into the methacrylate-modified support hydrogel, which was subsequently crosslinked by UV irradiation and finally removed.
Cell stimulators and implantable bioelectronics
An interesting hydrogel structure was synthesized by in-situ polymerizing acrylamide with dopamine and graphene oxide [36]. Polydopamine partially reduced graphene oxide converting it to the conductive graphene. The free catechol groups of polydopamine were responsible for self-healing and tissue adhesion. The hydrogel product could be used both as an adhesive electrode, motion sensor, as well as an in vivo implantable intramuscular electrode.
References
[1] W.A. Laftah, S. Hashim, A.K. Ibrahim. Polymer Hydrogels: A Review. Polymer-Plastics Technology and Engineering. 2011, 50, 1475-1486.
[2] E. Caló, V.V. Khutoryanskiy. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Pol. J. 2015, 65, 252-267.
[3] D. Lim, O. Wichterle. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118.
[4] S. Mantha, S. Pillai, P. Khayambashi, A.Upadhyay, Y. Zhang, O. Tao, H.M. Pham, S.D. Tran. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials (Basel) 2019, 12, 3323.
[5] M. Verhulsel, M, Vignes, S. Descroix, L. Malaquin, D.M. Vignjevic, J.-L. Viovy. A review of microfabrication and hydrogel engineering for micro-organs on chips. Biomaterials 2014, 35, 1816-1832.
[6] Y. Liu, S-h. Hsu. Synthesis and Biomedical Applications of Self-healing Hydrogels. Front. Chem. 2018, https://doi.org/10.3389/fchem.2018.00449.
[7] W. Wang, R. Narain, H. Zeng. Rational Design of Self-Healing Tough Hydrogels: A Mini Review. Front. Chem. 2018, https://doi.org/10.3389/fchem.2018.00497.
[8] H. Rammal, A.G. Nejad, A. Erdem, R. Mbeleck, M. Nematollahi, S.E. Diltemiz, H. Alem, M.A. Darabi, Y.N. Ertas, E.J. Caterson, N. Ashammakhi. Advances in biomedical applications of self-healing hydrogels. Mater. Chem. Front. 2021, 5, 4368-4400.
[9] S. Lü, C. Gao, X. Xu, X. Bai, H. Duan, N. Gao, C. Feng, Y. Xiong, M. Liu. Injectable and self healing carbohydrate-based hydrogel for cell encapsulation. ACS Appl. Mater. Interfaces 2015, 7, 13029–13037.
[10] V. Yesilyurt, M.J. Webber, E.A. Appel, C. Godwin, R. Langer, D.G. Anderson. Injectable self-healing glucose-responsive hydrogels with pH-regulated mechanical properties. Adv. Mater. 2016, 28, 86–91.
[11] L. He, D.E. Fullenkamp, J.G. Rivera, P.B. Messersmith. pH responsive self-healing hydrogels formed by boronate-catechol complexation. Chem. Commun. 2011, 47, 7497–7499.
[12] Q. Li, D.G. Barret, P.B. Messersmith, N. Holten-Andersen. Controlling hydrogel mechanics via bio-inspired polymer-nanoparticle bond dynamics. ACS Nano 2016, 10, 1317–1324.
[13] G.A. Barcan, X. Zhang, R.M. Waymouth. Structurally dynamic hydrogels derived from 1,2-dithiolanes. J. Am. Chem. Soc. 2015, 137, 5650–5653.
[14] M.H. Ghanian, H. Mirzadeh, H. Baharvand. In situ forming, cytocompatible, and self-recoverable tough hydrogels based on dual ionic and click cross-linked alginate. Biomacromolecules 2018, 19, 1646–1662.
[15] S. Li, L. Wang, X. Yu, C. Wang, Z. Wang. Synthesis and characterization of a novel double cross-linked hydrogel based on Diels-Alder click reaction and coordination bonding. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 299–309.
[16] X. Zhang, R.M. Waymouth. 1,2-Dithiolane-derived dynamic, covalent materials: cooperative self-assembly and reversible cross-linking. J. Am. Chem. Soc. 2017, 139, 3822–3833.
[17] X. Ye, X. Li, Y.Q. Shen, G.J. Chang, J.X. Yang, Z.W. Gu. Self-healing pH-sensitive cytosine- and guanosine-modified hyaluronic acid hydrogels via hydrogen bonding. Polymer (Guildf). 2017, 108, 348–360.
[18] D.C. Tuncaboylu, M. Sari, W. Oppermann, O. Okay. Tough and self-healing hydrogels formed via hydrophobic interactions. Macromolecules, 2011, 44, 4997–5005.
[19] S. Owusu-Nkwantabisah, J.R. Gillmor, S.C. Switalski, G. L. Slater. An autonomous self-healing hydrogel based on surfactant-free hydrophobic association. J. Appl. Polym. Sci. 2017, 134:44800, doi: 10.1002/app.44800.
[20] Y. Ren, R. Lou, X. Liu, M. Gao, H.Z. Zheng, T. Yang, H. Xie, W. Yu, X. Ma. A self-healing hydrogel formation strategy via exploiting endothermic interactions between polyelectrolytes. Chem. Commun. 2016, 52, 6273–6276.
[21] Y. Zheng, A. Hashidzume, Y. Takashima, H. Yamaguchi, A. Harada. Temperature-sensitive macroscopic assembly based on molecular recognition. ACS Macro Lett. 2012, 1, 1083–1085.
[22] H. Yamaguchi, Y. Kobayashi, R. Kobayashi, Y. Takashima, A. Hashidzume, A. Harada, Photoswitchable gel assembly based on molecular recognition. Nat. Commun. 2012, 3, 603.
[23] Y. Zheng, A. Hashidzume, A. Harada. pH-responsive self-assembly by molecular recognition on a macroscopic scale. Macromol. Rapid Commun. 2012, 34, 1062–1066.
[24] M. Nakahata, Y. Takashima, H. Yamaguchi, A. Harada, Redoxresponsive self-healing materials formed from host-guest polymers. Nat. Commun. 2011, 2, 511.
[25] C. B. Rodell, A.L. Kaminski, J.A. Burdick. Rational design of network properties in guest–host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules 2013, 14, 4125–4134.
[26] L. Li, B. Yan, J. Yang, L. Chen, H. Zeng. Novel mussel-inspired injectable self-healing hydrogel with anti-biofouling property. Adv. Mater. 2015, 27, 1294–1299.
[27] M. Krogsgaard, A. Andersena, H. Birkedal. Gels and threads: mussel-inspired one-pot route to advanced responsive materials. 2014, 50, 13278-13281.
[28]. P. Casuso, A. Perez-San Vicente, H. Iribar, A. Gutierrez-Rivera, A. Izeta, I. Loinaz, G. Cabañero, H.-J. Grande, I. Odriozola, D. Dupin. Aurophilically cross-linked “dynamic” hydrogels mimicking healthy synovial fluid properties. Chem. Commun. 2014, 50, 15199–15201.
[29] G.H. Deng, F.Y. Li, H.X. Yu, F.Y. Liu, C.Y. Liu, W.X. Sun, H. Jiang, Y. Chen. Dynamic hydrogels with an environmental adaptive self-healing ability and dual responsive sol-gel transitions. ACS Macro Lett. 2012, 1, 275–279.
[30] L.L. Wang, C.B. Highley, Y.C. Yeh, J.H. Galarraga, S. Uman, J.A. Burdick. Three-dimensional extrusion bioprinting of single- and double network hydrogels containing dynamic covalent crosslinks. J. Biomed. Mater. Res. 2018, A 106, 865–875.
[31] R. Xing, K. Liu, T. Jiao, N. Zhang, K. Ma, R. Zhang, Q.Zou, G. Ma, X. Yan. An injectable self-assembling collagen-gold hybrid hydrogel for combinatorial antitumor photothermal/photodynamic therapy. Adv. Mater. 2016, 28, 3669–3676.
[32] W. Xie, Q. Gao, Z. Guo, D. Wang, F. Gao, X. Wang, .Y. Wei, L. Zhao. Injectable and self-healing thermosensitive magnetic hydrogel for asynchronous control release of doxorubicin and docetaxel to treat triple-negative breast cancer. ACS Appl. Mater. Interfaces 2017, 9, 33660–33673.
[33] J.Y. Wang, D. Wang, H. Yan, L. Tao, Y. Wei, Y. Li, X. Wang, W. Zhao, Y. Zhang, L. Zhao, X. Sun. An injectable ionic hydrogel inducing high temperature hyperthermia for microwave tumor ablation. J. Mater. Chem. 2017, B 5, 4110–4120.
[34] A.C. Gaffey, M.H. Chen, C.M. Venkataraman, A. Trubelja, C.B. Rodell, P.V. Dinh, G. Hung, J.W. MacArthur, R.V. Soopan, J.A. Burdick, P. Atluri. Injectable shear-thinning hydrogels used to deliver endothelial progenitor cells, enhance cell engraftment, and improve ischemic myocardium. J. Thorac. Cardiovasc. Surg. 2015, 150, 1268–1276.
[35] P.Y. Dankers, T.M. Hermans, T. W. Baughman, Y. Kamikawa, R.E. Kieltyka, M.M. Bastings, H.M. Janssen, N.A.J.M. Sommerdijk, A. Larsen, M.J.A. van Luyn, A.W. Bosman, E.R. Popa, G. Fytas, E.W. Meijer. Hierarchical formation of supramolecular transient networks in water: a modular injectable delivery system. Adv. Mater. 2012, 24, 2703–2709.
[36] C.B. Highley, C.B., Rodell, J.A. Burdick. Direct 3D printing of shear thinning hydrogels into self-healing hydrogels. Adv. Mater. 2015, 27, 5075–5079.
[37] L. Han, X. Lu, M. Wang, D. Gan, W. Deng, K. Wang L. Fang, K. Liu, C.W. Chan, Y. Tang, L.-T. Weng, H. Yuan. A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogels cell stimulators and implantable bioelectronics. Small 2017, 13:1601916; doi: 10.1002/smll.201601916.