Recycling polymeric materials via an alkaline hydrolysis depolymerization process: gr3n approach
This article is sponsored by Gr3n.

Abstract
Polyethylene terephthalate (PET) is one of the most widely used polymers all around the world. At the end of its life, it is mechanically recycled or landfilled/incinerated. PET wastes not mechanically reused can be depolymerized via chemical processes to recover the monomers than can be used to produce new “virgin” PET. gr3n developed a revolutionary chemical process to recycle post-consumer waste from several types of PET products (like bottles, food containers and polyester clothes) in a closed-loop cycle, obtaining as output pure terephthalic acid (TPA) and mono ethylene glycol (MEG) that currently feed the PET production cycle. Customers are PET producers pushed by global brands, who make extensive use of PET and that are embracing greener policies, as well as municipalities and recyclers.
1. Polyester overview
1.1 Global PET market and availability of advanced recycling’s raw materials
PET is the second most used polymer in the world after Polyethylene. The global PET market is worth around 110 B€, 27 B€ of which go to packaging (expected to reach 35 B€ by 2023) and 80 B€ to polyester (growing 8.0% year over year up to 2024). Translated into volumes, the expected global PET demand by 2022 is around 80 million metric tons (30% bottles, 70% polyester), growing at a 4% rate year of year. Such trends are important for advanced recycling, as they both represent the global demand and give an indication of the expected availability of the raw material used for the process.
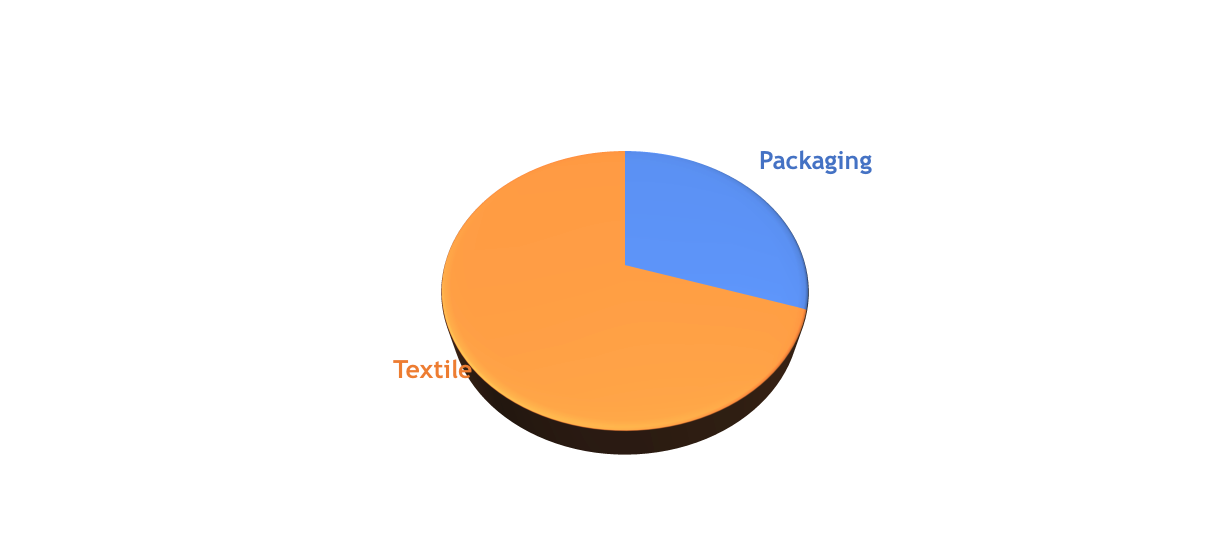
Figure 1. The main market for polyester is textile, although the packaging value chain is more developed
In fact, advanced recycling’s feedstock is any PET-based post-consumer waste, as bottles, trays and textiles, in an amount that can vary quite a lot depending on the technology: each year 70 B€ - 110 B€ of plastic packaging material is wasted from the economy. If we only consider PET bottles and packaging, 25 million tons/year are recovered globally, around 85% of which can hardly be recycled. As an example, around 1 Mt/y of European PET waste recovered is composed of colored PET baled bottles, which have a very limited market value compared to colorless. The situation is even worse if we also take into consideration polyester, because it cannot be treated and is sent to landfills or incineration only.
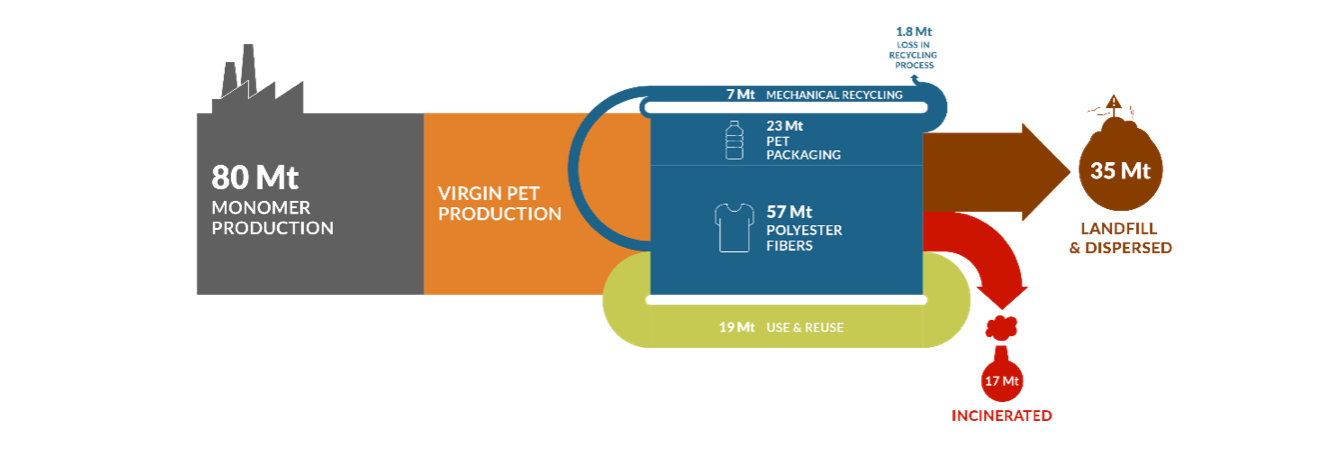
Figure 2. The polyester lifecycle: from monomers production to disposal
Hard data are the best way to describe how plastics are challenging our eco-system:
- In 2017, 348 million tons of plastics were produced globally [1], and the production is expected to double again over the next 20 years [2].
- at least 8 million tons of plastics (equivalent to 350,000 filled shipping containers) leak into oceans every year, adding to the current burden of 150 million tons [3].
- 33% of plastics are used only once, and then thrown away with only 8% being recycled and converted to low-value products [4] that cannot be recycled again.
- 80 million tons of PET (the second most used plastic polymer) are produced each year from petroleum refining. The same year, 52 million tons waste is landfilled, incinerated or floats into seas and oceans.
Such high rate of production and low rate of re-use and recycling has brought us to the point where in just over 100 years synthetic plastics have gone from being essentially non-existent to one of the biggest environmental and social problems faced by the planet today.
1.2 European regulations
In 2018 the EU Commission launched the European Strategy for Plastics [5], which spurred the development of several initiatives such as the ban of single-use plastics from 2021 (“Single-use Plastics Directive Proposal” [6]), the birth of the Circular Plastic Alliance [7] to foster the market of recycled plastics in Europe, and the 2018 campaign calling stakeholders to submit voluntary pledges for recycled plastics. Different countries are setting targets and tax programs to control recovery and recycling of PET. For example, Sweden has a deposit program for bottles where end users pay and are then refunded by putting the used bottles into recycling machines. Germany is activating various recycling initiatives, where manufactures also pay a fee based on weight and material type. In Europe, plastic producers are charged with an environmental contribution for plastic packaging.
A new EU legislative framework on waste has entered into force in July 2018 setting clear targets for reduction of waste and establishing an ambitious and credible long-term path for waste management and recycling. Key elements include a common EU target for recycling 70% of packaging waste by 2030 while separate collection obligations are strengthened and extended to textiles (by end 2025).
The European Commission initiatives are placing EU as a world leader in plastic management and sustainable alternatives to avoid marine pollution, as recently remarked by President Ursula von der Leyen in her speech about the adoption of the European Green Deal. At the same time, also the US are embracing greener environmental policies, moving from only 3 cities with single-use plastic ban in 2008 to 388 in 2018 and more than 2100 forecasted from 2030.
1.3 PET production: from monomers to pellets
The production of PET starts with the extraction of crude oil. This non-renewable fossil fuel resource consists of thousands of different organic compounds, including pure hydrocarbons, and molecules with functional groups containing oxygen, nitrogen, sulfur and certain minerals.
There are two main methods for removing fossil fuels from the ground: mining and drilling. Mining is used to extract solid fossil fuels, such as coal, by digging, scraping, or otherwise exposing buried resources. Drilling methods help extract liquid or gaseous fossil fuels that can be forced to flow to the surface, such as conventional oil and natural gas. Both processes carry serious health and environmental impacts, as consumes energy and disrupts the surrounding ecosystem.
Because crude oil is such a complex mix, it must be refined and processed to obtain the building blocks of PET, i.e., ethylene glycol (EG) and terephthalic acid (TPA). This is achieved by heating, distillation and other processes that release harmful toxins such as BTEX compounds (benzene, toluene, ethylbenzene and xylene), particulate matter, nitrogen oxides (NOx), SO2 and CO. If not controlled, these compounds can contribute to air pollution and global warming. Furthermore, oil and the chemicals used during extraction are often spilled.
The building blocks for PET can also be obtained from recycled materials or renewable resources such as CO2 and biomass. Given the abundance of CO2 and the threat it poses, carbon capture and utilization are now considered not only viable but possibly essential for future value chains. Laboratory-scale electrochemical systems can efficiently convert CO2 into chemical building blocks (such as ethylene glycol) to obtain polymers but more research and development is required to optimize and scale up this technology. Whereas CO2 conversion technology is not yet mature, ethylene glycol has been produced from biomass for many years, and industrial biobased processes for the production of TPA are emerging [8]. However, the economic feasibility of biobased production is currently limited. As a consequence, less than 1% of PET production in 2018 was partially biobased, meaning that ethylene glycol was derived from biobased sources, but TPA was still produced from oil.
Key recommendations to improve the sustainability of polyester manufacturing at the raw material stage therefore include phasing out the use of fossil fuels as a material source for PET production and for the provision of energy. The raw materials can be replaced with recycled chemicals and/or renewable feedstocks, depending on which has the smallest environmental footprint (verified through LCA) and the energy requirements can be provided by renewable sources.
2. Plastic recycling
The most common method for the recycling of plastic waste is mechanical recycling. This process typically includes collection, sorting, washing and grinding of the material. Steps may occur in a different order, multiple times or not at all, depending on the origins and composition of the waste. Some examples:
- Post-industrial materials: this is clean waste of a well-known composition. It does not require collection (can be re-used in the same company), sorting (it is a mono-stream) or washing (the material is clean);
- Mixed post-consumer packaging waste: the bigger amount of plastics waste, this stream requires collecting (from the consumer), sorting (for example select out the PET bottles), washing (removal of contaminants) and then grinding, often followed by regranulation (in this case only transparent and lightly colored bottles. and only 10% of global consumption is recycled. Furthermore, a breakdown of polymer chains occurs when the resin goes through multiple mechanical cycles, degrading its intrinsic viscosity and limiting the number of times the process can be repeated).
Although a broad variation in recycling and recovery rate of waste plastics exists within Europe, almost 8 Mt of plastics waste were still landfilled in Europe in 2014. This amount of plastics corresponds to almost 100 million barrels of oil [9]. Worldwide, the amount of plastics ending up in landfill is almost half of the produced amount, being 150 Mt annually. This amount of plastics is quite considerable; hence it has high potential to be used as feedstock for the production of valuable products or to be used for energy recovery. The latter being less favorable from an environmental point of view, the energy content of plastic is nonetheless comparable with heating oil, respectively 42.6 MJ/l and 443.5 MJ/kg. Hence, a cheap source of energy can be found in using them as secondary fuel. However, incineration of plastics introduces the need of advanced pollution control measures, highly regulated by the EU Hazardous Waste Incineration Directive. Energy recovery of plastic waste yields toxic and noxious dioxins that should be carefully monitored. Processing difficulties such as those caused by the use of coatings and paints complicate the process of mechanical recycling. Also, contaminants can be not completely soluble and can induce phase separation, with a negative impact on the mechanical properties as a result.
2.1 Chemical recycling of polyester
Chemical recycling is the broad term used to describe a range of emerging technologies in the waste management industry which allow plastics to be recycled, that are difficult, impossible or uneconomic to recycle mechanically. The goal is to be able to upcycle the material, so to recycle it in a way that the resulting product is of a higher value than the original item, and in particular to create an object of greater value from a discarded object of lesser value.
Polyethylene terephthalate (PET) is a thermoplastic polymer synthesized through a polycondensation reaction between mono ethylene glycol (MEG) and terephthalic acid (TPA), or through a transesterification reaction between dimethyl terephthalate (DMT) and MEG.
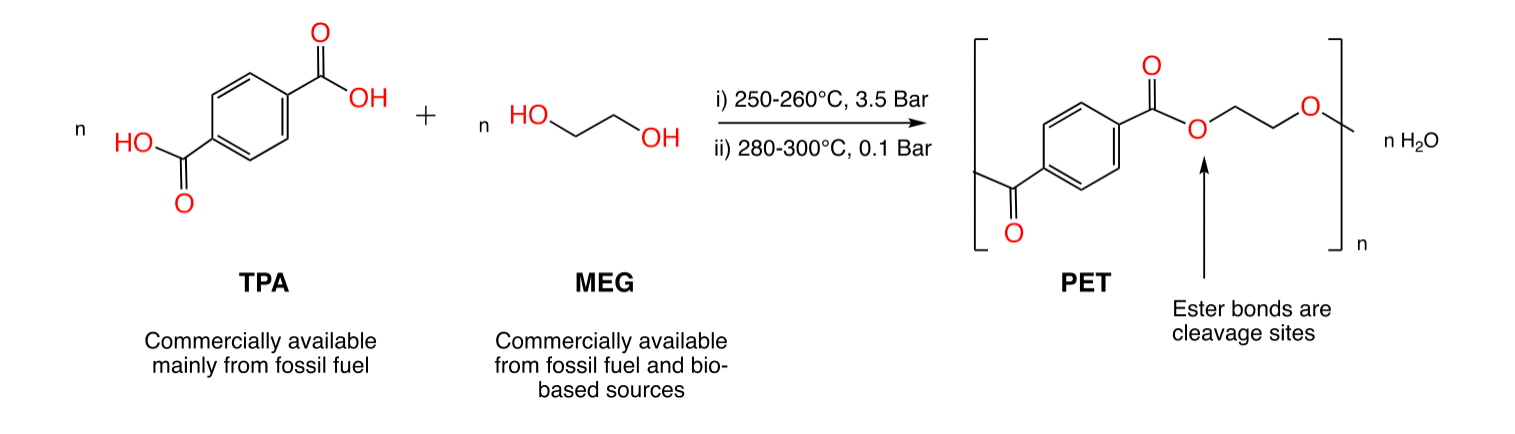
Figure 3. Synthesis of PET starting from TPA and MEG.
It means it is possible to chemically recycle in different ways:
- Pyrolysis
- Methanolysis
- Glycolysis
- Hydrolysis
2.1.1. Pyrolysis
The pyrolysis of PET (normally at > 450°C) yields TPA and oligomers thereof, which can be further hydrolyzed to obtain the TPA monomer. Pyrolysis generally leads to other liquid and gaseous side-products, reducing process efficiency, and necessitating costly separation steps. Although interesting, this is probably not the best option for polyesters, but it can be largely applied got other polymers like polyolefins.
2.1.2. Methanolysis
Another method to depolymerize PET into its monomers is through methanolysis. In this process, methanol reacts with PET at high temperatures (180–280 °C) and pressures (20–40 atm) in the presence of a catalyst. This reaction leads to the formation of DMT and EG, which can then be used to resynthesize PET through a transesterification reaction. A major drawback of this method, outside of the high temperatures and pressures (there are new technologies that are working at low temperature and pressure), is again the purification process. The crude product contains not only DMT and EG but also other alcohols and phthalate derivatives.
2.1.3. Glycolysis
Glycolysis of PET is an area that has been widely studied. This is a very versatile process due to the various potential applications of the products obtained. In this process, PET is depolymerized by glycols to form monomers, oligomers, and/or polyols, which can then be used for different applications. Some of the glycols that have drawn particular recent interest for this application include ethylene glycol, diethylene glycol (DEG), propylene glycol, butylene glycol, and dipropyleneglycol (DPG). If EG is used to depolymerize PET, the major product formed is bis(2-hydroxyethyl) terephthalate (BHET), which can be used to synthesize PET. A challenge in these promising reactions is the immiscibility of PET with the polyols. gr3n process: a new way to approach chemical recycling
2.1.4. Hydrolysis
Another chemical process that has shown great promise for the depolymerization of PET into its monomeric units is hydrolysis (reaction with water at elevated temperatures and/or with a catalyst). The products yielded from this method are TPA (or a terephthalate salt)and MEG. There are three different types of hydrolysis that have been studied in greatest detail: acidic, alkaline, or neutral hydrolysis. The most common acid used is sulfuric acid. Although the yields obtained from this method are generally high, separation of the EG from the highly acidic solution is a major drawback of this method.
Alkaline hydrolysis generally employs aqueous solutions of 4–20 wt% NaOH. This process yields the sodium terephthalate and MEG in relatively good yields, up to 100% PET conversion. However, longer reaction times (3–5 hr) and high temperatures (> 200 °C) than needed for acidic hydrolysis techniques are notable drawbacks of this method. Neutral hydrolysis, without the need for stoichiometric acid or base, would be ideal, but these processes generally produce low purity monomers and have relatively slow rate of reaction.
2.2. gr3n approach
If PET plastic products were consistent in their resin composition, color, transparency, weight and size, everything would be easier, as waste plastics could be recycled all together (like aluminum, which shows the highest rates of recycling globally). On the contrary, with millions of different PET/polyester plastic products and packages on the market, clearly this is not the case.
gr3n proposes a completely new way to break this vicious circle and create virgin plastic from waste. Our vision is to enable the first truly circular approach to PET recycling, empowered by our patented technology able to break any kind of PET/polyester into its two main building blocks, for creating new virgin-like PET plastics and polyester infinitely and cost-effectively.
2.2.1 Our process
We are feeding the process with two things: post-industrial and/or post-consumer feedstock, both from packaging and textile, and energy, as we are running a chemical reaction. The first step is grinding the materials, reducing it into smaller pieces, in order to increase the surface area of the material and therefore the contact surface with the solvolytic mixture. The solvolytic mixture consists of an alkaline hydroxide in ethylene glycol (MEG), and is prepared in a mixing unit.
The ground material coming from the grinding unit, and the solvolytic mixture are mixed to form a heterogeneous reaction mixture and left to react in a microwave reactor. Microwaves with frequency between 300 MHz and 300 GHz and power density between 1 W/L and 1000 W/L are sent into the microwave chamber. The reaction with the solvolytic mixture of NaOH (or KOH or other alkaline hydroxide) in ethylene glycol produces a solution of terephthalic acid (TPA) and/or its salts (for example, sodic or potassic salt) in ethylene glycol. Advantageously, the reaction temperature is between approximately 150 and approximately 350°C and the pressure between approximately 1 atm and approximately 20 atm. The reaction is quite fast (i.e., around 10 minutes).
The distillation unit is fed with the reaction products coming from the filtering unit (comprising mainly a solution of terephthalic acid and/or its salts in ethylene glycol) and produces ethylene glycol, a solution of terephthalic acid and/or relative salts, and waste products.
The precipitation unit receives a solution containing terephthalic acid to be recovered, obtained from the distillation unit, to which water is added. The solution is treated in the precipitation unit with mineral acids (i.e HCI) until achieving a pH of approximately 2÷3, thus obtaining precipitation of the terephthalic acid; the terephthalic acid is removed, in the filtering and/or centrifugation unit, from the acid aqueous solution and then sent to the washing unit and to the drying unit. Terephthalic acid is obtained with a purity level sufficient to undergo a new polymerization reaction.
The aqueous acid solution resulting from the removal of the terephthalic acid is neutralized in the neutralization unit with alkaline bases, up to neutral pH; the resulting salts, are used in the electrolysis unit for an electrolytic chloralkali process.
With this approach we can close the textile to textile loop, but also we can close a smaller loop in our own process, avoiding to feed the process every time with new chemicals.
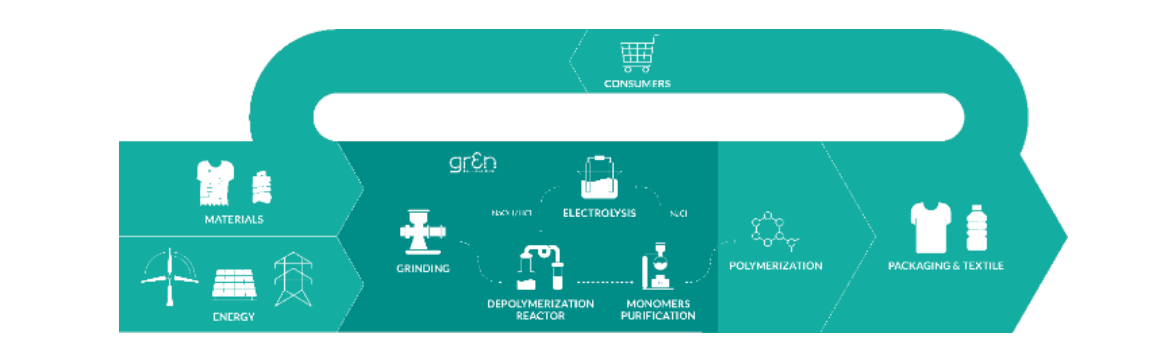
Figure 4. gr3n process as a part of the new polyester lifecycle.
Compared to other companies that are working in the chemical recycling of PET, gr3n can leverage the following competitive advantages:
- cPET technology can use feedstock with high level of contaminants (up to 30%), drastically lowering production cost.
- The process does not require any catalyst to run.
- The** chemicals needed for the process are produced on site** from the by-product of the process (NaCl): no disposal is needed apart from the contaminants entering the process along with PET.
- Thanks to its unique design, the reaction is fast and all the reactors are in-line, avoiding the slowdowns due to batch reactors and leading to high production rate.
- Monomers produced are the state of the art for PET production.
- Plant economic sustainability is guaranteed even for low-volume production (30,000 ton/y), thus allowing the building of small local production facilities, tailored on the specific needs of the post-consumer recovery network of regional municipalities.
- Thanks to the engineering approach used to design the microwave reactor based on several parallel reactive chambers, our technology is modular. This allows to enhance the scalability of the licensing model, easily combining our package with other sub-systems (i.e. grinding; vaporization; electrolysis), reducing the overall schedule by about 4-6 months and reducing final cost for client.
3. Towards a new ecosystem
The value chain related with packaging recycling, and in particular with PET bottles, is quite developed: Mechanical recycling can manage colorless and light blue material without problems, so there is already a market behind it, and the feedstock is then already valorized. Some of the companies are able to reuse their post-industrial waste but this is just a small amount compared to the whole amount of PET employed. Some examples are PET films used as support for other application, colored films, opaque bottles, multilayers materials, etc., that it is not possible/inconvenient to recycle via mechanical recycling.
There is a huge difference when the feedstock is textile, as there is no such a structured behind collection, sorting and recycling. Although there is already mechanical recycling in place for textile, there are more or less the same limitations for packaging, as it is not possible to manage highly colored textiles, etc. The result is a product downcycling, which you can use for lower value applications, maybe with some additives.
Our solution is P-Turn, a program that is organized in four phases: Mapping, Building, Connecting, Closing. The goal is to allow everyone who has feedstock available to provide us with their material in the most efficient way. We will recycle the material, avoiding incineration and landfill. This means the stakeholders will be able to reduce their carbon footprint by leaving the carbon into the loop: this means reducing CO2 emission and saving non-renewable sources.
Here a brief description of each phase:
- Mapping the feedstock: We would like to know where the material is, to help facilitate the process when you will need to dispose your waste. The first phase will be a voluntary disclosure of the post-industrial and post-consumer feedstock that you have on your premises. If we know where is, we can pick it up.
- Building the network: The intention of the program is to create a credible path to simplify the interaction between us. It is necessary to establish a structured and solid network through a one-to-one relationship between your company and gr3n. All the information provided by you will be treated as confidential and will not be disclosed to any other party.
- Connecting the dots: At this stage sit back and watch gr3n work for you. With the information you have provided us, we will coordinate the whole value chain, from material collection till depolymerization and further re-polymerization.
- Closing the loop: Everything is now set, so you will receive all the necessary information to move forward, e.g. where to ship your material, when, etc. What's next? Sit down, relax, and enjoy closing the loop.
Join P-Turn program is pretty simple: you have just to go to https://p-turn.gr3n-recycling.com/join.html , complete the form providing us with your data, and create a simple login.
Figure 5. P-Turn registration form
As this is a one-to-one relationship between the customer and gr3n, all the data collected will be managed securely by gr3n. At the end of each phase, we will provide timeous information on how everything is progressing.
Figure 6. P-Turn Login form
References
[1] Statista. Global plastic production from 1950 to 2017. (2019). Available at: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/
[2] Plastics-Europe. Plastics-the Facts 2018 An analysis of European plastics production, demand and waste data. (2018).
[3] Lebreton, L. et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 8, 4666 (2018).
[4] World Economic Forum. The New Plastics Economy. (2016)
[5] http://europa.eu/rapid/press-release_IP-18-5_en.htm)
[6] https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A52018PC0340
[7] http://europa.eu/rapid/press-release_IP-18-6728_en.htm
[8] F. Wang, Z. Tong, ChemistrySelect 2016, 1, 5538.
[9] Plastics Europe, 2015