Quantitative removal of water pollutants: EDTA mimic polyamidoamine resin as reversible absorber of heavy metal ions
Environmental pollution by heavy metal ions is of great concern worldwide as these substances can be harmful to the environment and human health [1]. Some heavy metal ions affect cell functions, others accumulate in various organs causing serious diseases, including cancer. Heavy metal ions are discarded in waters, soils and atmosphere due to improper waste disposal in agriculture and industrial activities, and the use of fertilizers and pesticides. National regulations set strict limits for the presence of heavy metal ions in water for both civil and industrial uses. In circumstances where these limits are exceeded, these pollutants must absolutely be removed from the environment. A multifunctional polyamidoamine-based cross-linked resin proved capable of rapidly, quantitatively and reversibly absorbing numerous heavy metal ions, namely Cu2+, Cd2+, Pb2+, Zn2+, Ni2+, Co2+ [2]. This resin was easily regenerated by treatment with aqueous acid solutions.
Objective
The project’s objective was to design eco-friendly absorbing resins obtained through a water-based process capable to fast, quantitatively and reversibly absorb heavy metal ions even from very low concentration solutions. For this purpose, a multifunctional highly water-swellable cross-linked polyamidoamine-based resin was synthesized, whose structure resembled ethylenediaminetetraacetic acid (EDTA), a small molecule well known for its exceptional complexing capacity towards heavy metal ions. It is worth mentioning here that also linear polyamidoamines (PAAs) carrying normally at least two amines per repeat unit that neither belong to a cyclic structure nor are separated by more than three carbon atoms form stable coordination complexes with heavy metal ions [3].
Synthesis of the LMT85 PAA cross-linked resin
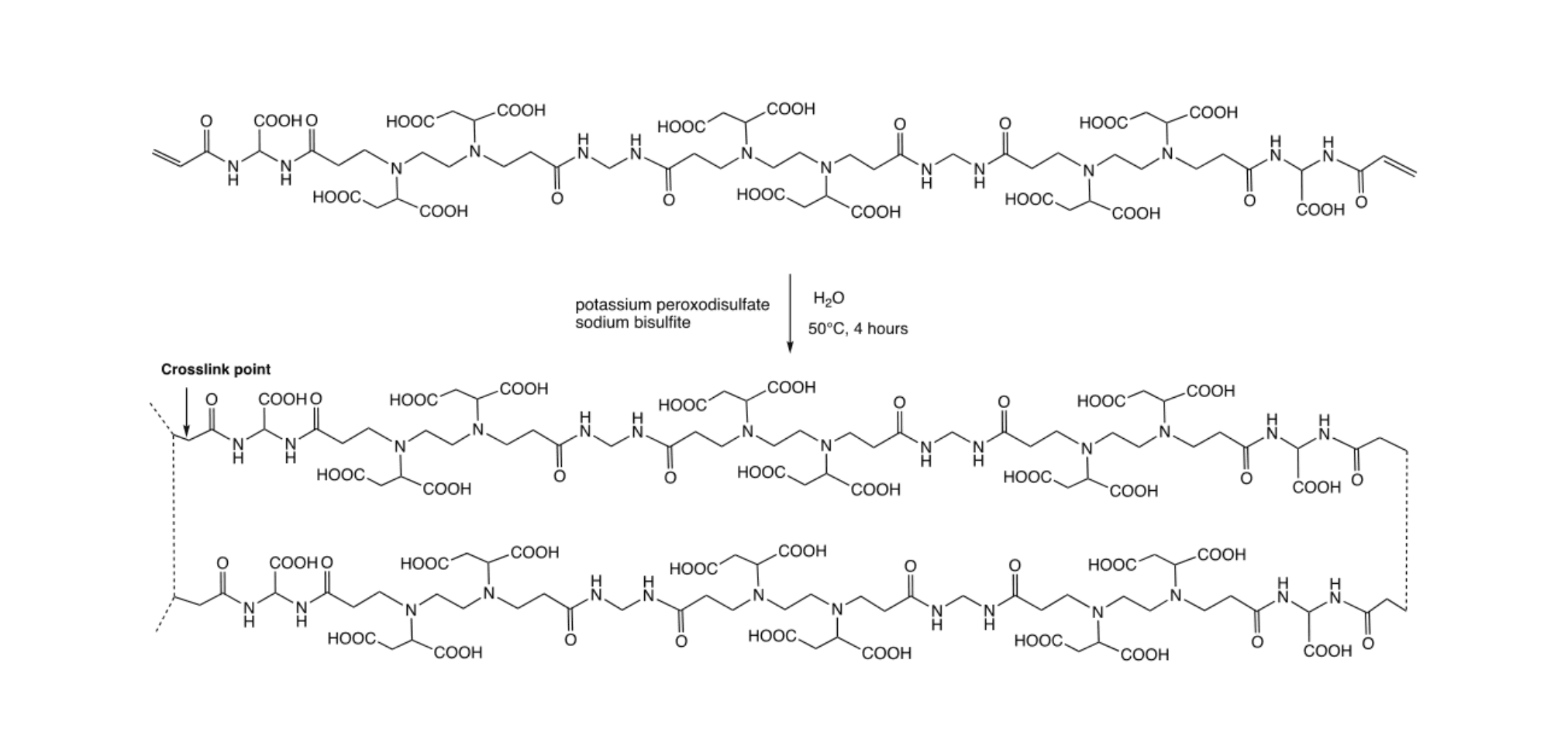
The EDTA mimic resin coded LMT85 was obtained by a two-step procedure. In the first step (Scheme 1), a low molecular weight α,ω-acrylamide-end-capped PAA oligomer was synthesized by reacting ethylenediamine-N,N′-disuccinic acid with a 50:50 w/w mixture of bisacrylamides, namey 2,2-bis(acrylamido)acetic acid and N,N’-methylene bis(acrylamide), in water and in the presence of enough sodium hydroxide to neutralize all the carboxyl groups present in the monomers. The reaction mechanism was a stepwise aza-Michael polyaddition of amines with the 1,4-unsaturated acrylamides. The overall ratio of the acrylamide/amine functions was 1.4:1, corresponding to a theoretical number average polymerization degree of 6. The reaction mixture was allowed to react first 2 hours at 50°C then 48 hours at 25°C in the dark and under nitrogen atmosphere.
Scheme 1. Synthesis of the α,ω-acrylamide terminated PAA oligomer. For the sake of simplicity, all carboxylic groups have been represented in their protonated form.
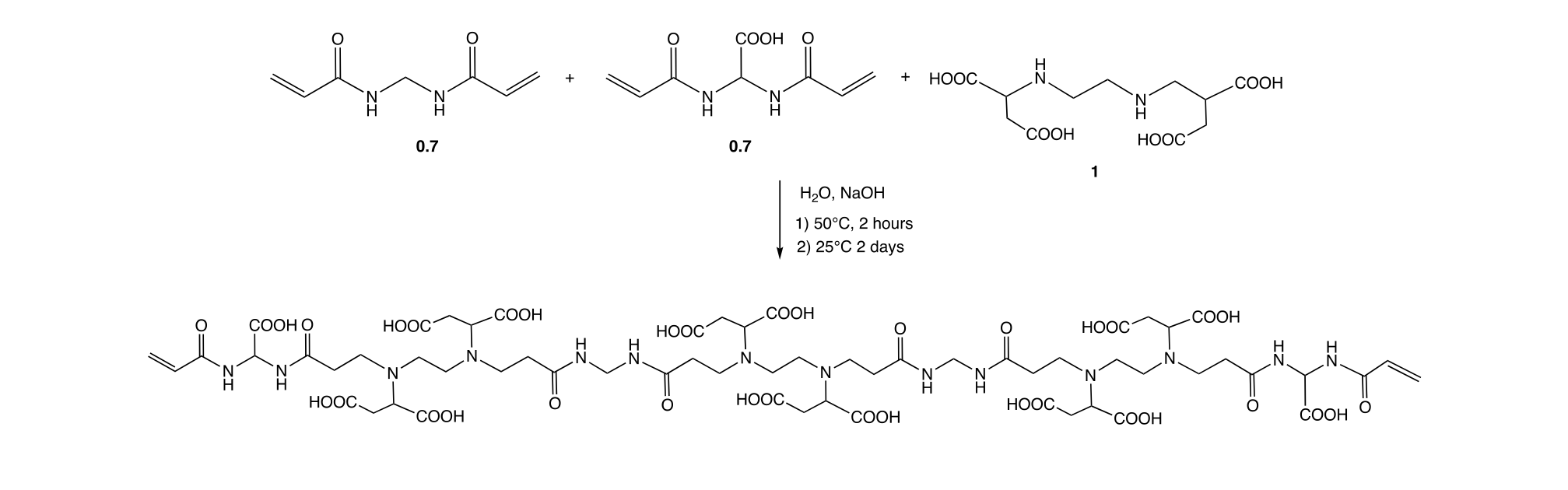
In the second step (Scheme 2), the oligomeric precursor obtained in step 1 was polymerized by radical mechanism using the potassium peroxodisulfate – sodium bisulfite redox pair as initiator at pH 5.5. The final product was a transparent hydrogel, which was finely ground, which was extensively washed by repeated deswelling/swelling cycles obtained by ethanol (deswelling) followed by water (swelling) treatments.
The LMT85 water uptake ratio, calculated as weight of water absorbed per weight of dry resin was about 35, whereas the ethanol uptake was minimal, clearly confirming the high hydrophilicity of this resin.
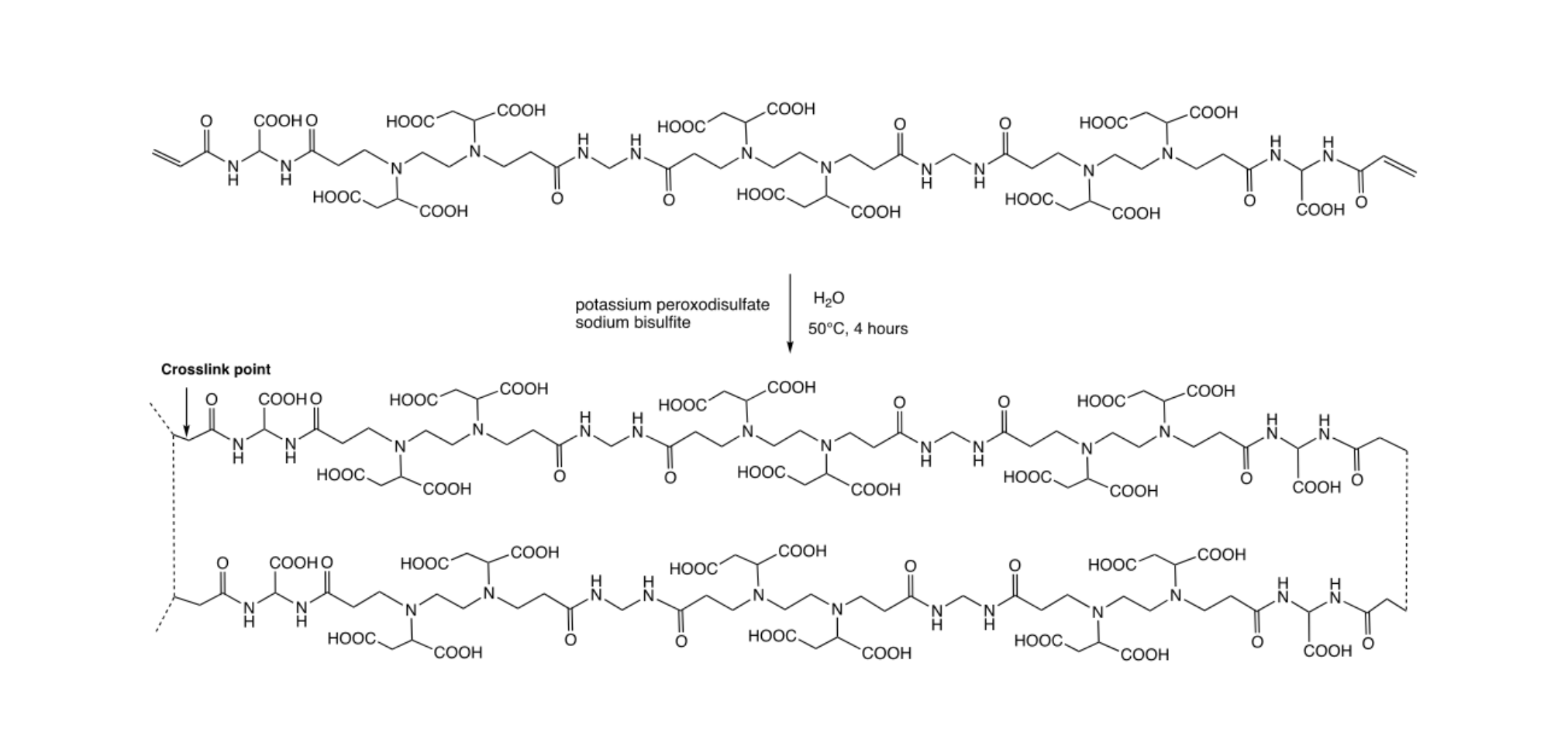
Scheme 2. Synthesis of the LMT85 resin from the α,ω-acrylamide terminated PAA oligomer.
Experimental set up
In this study, a set of divalent heavy metal ions was used as a benchmark, namely Cu2+, Cd2+, Pb2+, Zn2+, Ni2+, Co2+, corresponding to the DIN 38406-16 protocol for the voltammetric determination of heavy metals in tap waters [4]. The absorbent resin was confined in a porous paper bag dipped in a water solution of the ion to be analyzed, so that it did not contaminate the electrodes and could be easily removed (Figure 1).
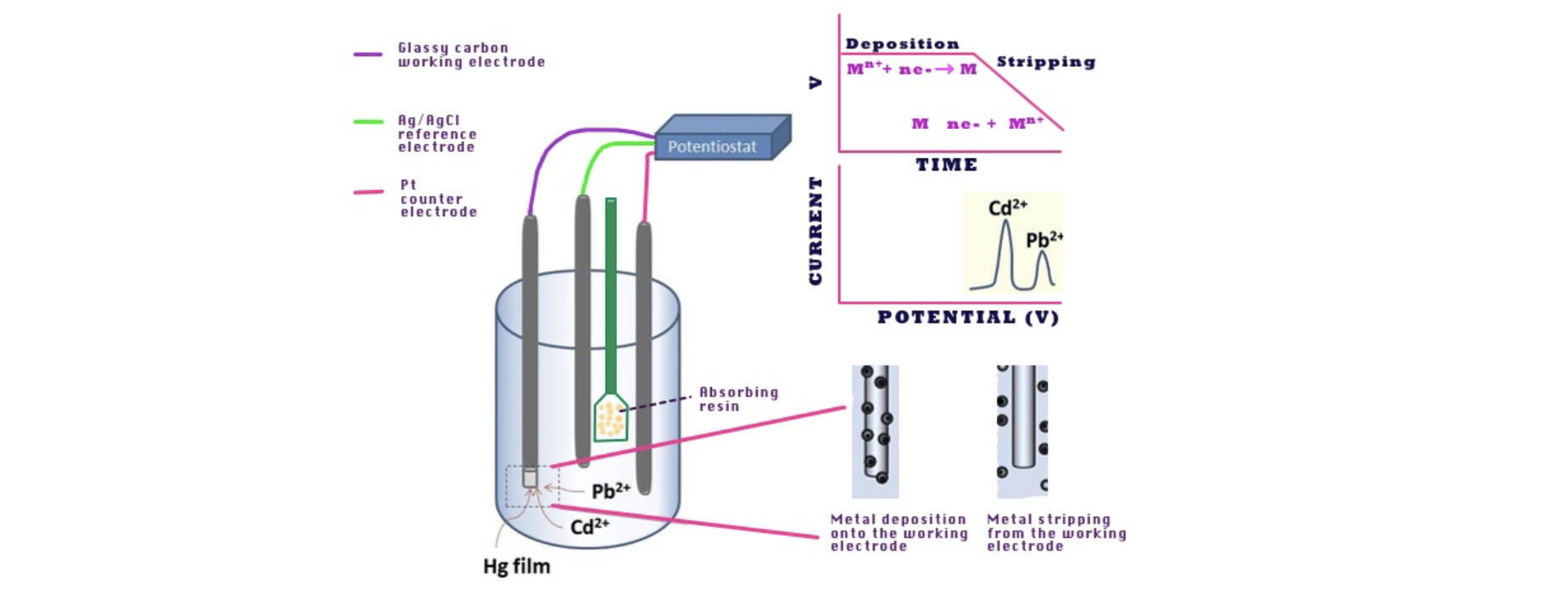
Figure 1. Experimental set up used for monitoring heavy metal ion absorption by cyclic voltammetry.
The kinetics of the heavy metal ion absorption by the LMT85 resin was studied by in situ monitoring the decrease with time of the voltammetric peak current of the solution, which was proportional to the ion concentration, on a working hanging mercury drop electrode. Square wave voltammetry (SWV) was adopted for intermediate to low concentrations. Anodic stripping voltammetry (ASV), implying local preconcentration of the metal by ion reduction to metal amalgam in controlled conditions followed by differential pulse voltammetry (DPV) reoxidation [5], was adopted for Cu2+, Cd2+, Pb2+, and Zn2+ at the lowest concentrations (Figure 1). The operating concentrations were normally in the 10-4 M to 10-5 M range. The typical pH value of tap or drinking waters, that is, pH 6.8 was chosen as a reference pH value. In all instances but Pb2+, a phosphate buffer could be used to ensure constant pH level. Many measurements were duplicated using TRIS buffer.
The maximum absorption capacity of the two resins in saturation conditions has been evaluated for each metal ion equilibrating for 3 days under stirring 100 cm3 of a 0.001 M solution of the selected ion, with nitrate counter anion, with a metal ion/resin repeating unit ratio of 3 : 1. Otherwise, typical resin repeating unit: Me2+ molar ratios varied in the range 5:1 to 50:1.
Absorption kinetics
The LMT85 resin apparently absorbed all ions with comparable speed and efficiency (Figure 2). The absorption was quantitative, within the sensitivity limit of the technique, even starting from 5 10-5 M (3 ppm) concentration. The absorption process could be reversed by acidifying with sequential additions of aliquots of a nitric acid solution (Figure 3), suggesting a potential for metal recover and/or resin recycle after use.
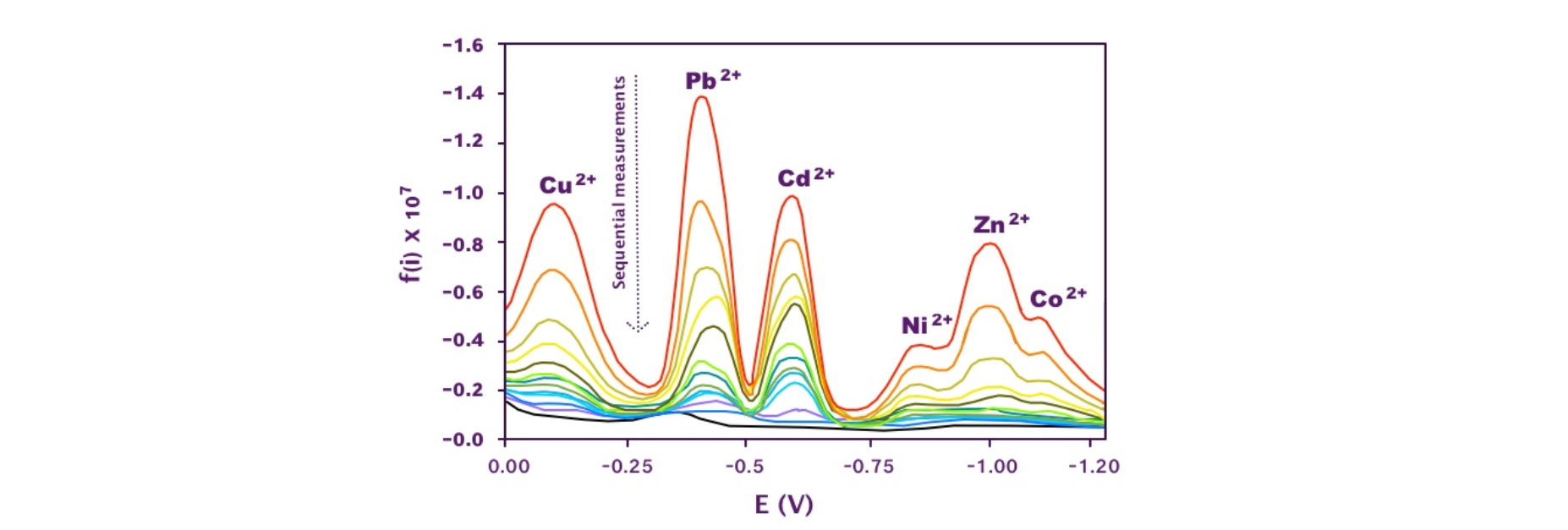
Figure 2. Absorption tests on LMT85 in multiple heavy metal ion solution with starting concentration 0.000025 M. The different cyclovoltammetry curves correspond to sequential measurements. Peak reduction was observed with time, due to the reduction of the ion concentration in the aqueous solution.
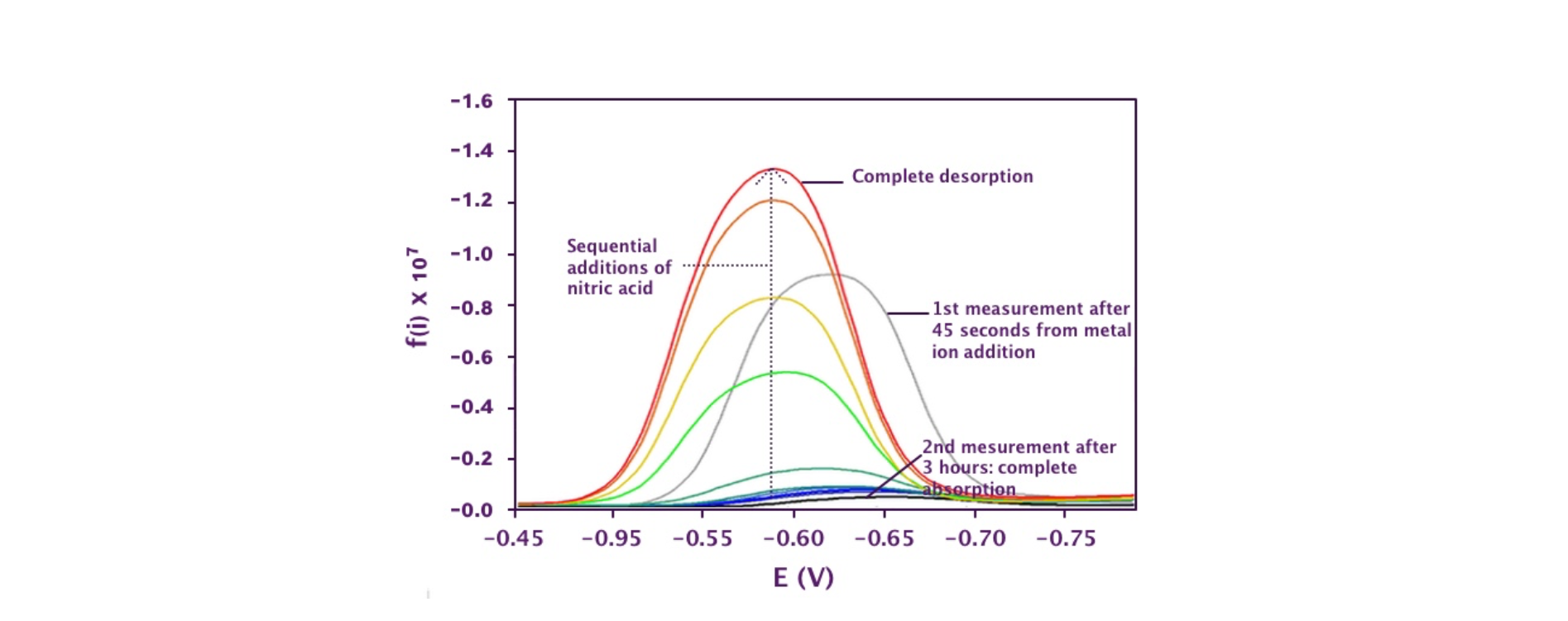
Figure 3. Release of Cd2+ from LMT85 upon progressive acidification by addition of small aliquots of concentrated nitric acid after complete metal absorption.
The absorption kinetics, carried out with single ion solutions, were found to fit a pseudo-second order model, implying the following

where qt is the absorption capacity of the metal ion at time t (in mmol g-1), qe the same quantity at the equilibrium, while k2 is the pseudo-second order constant in or g mmol-1 min-1. Rearranging and integrating equation (1) gives the following

that can be used for linearization of the kinetic characteristics. According to equation (2) the halving time is

Table 1 summarizes the kinetic data relative to heavy metal ion absorption by the LMT85 resin.
Table 1. Kinetic parameters for heavy metal ion absorption by LMT85 in different experimental conditions.
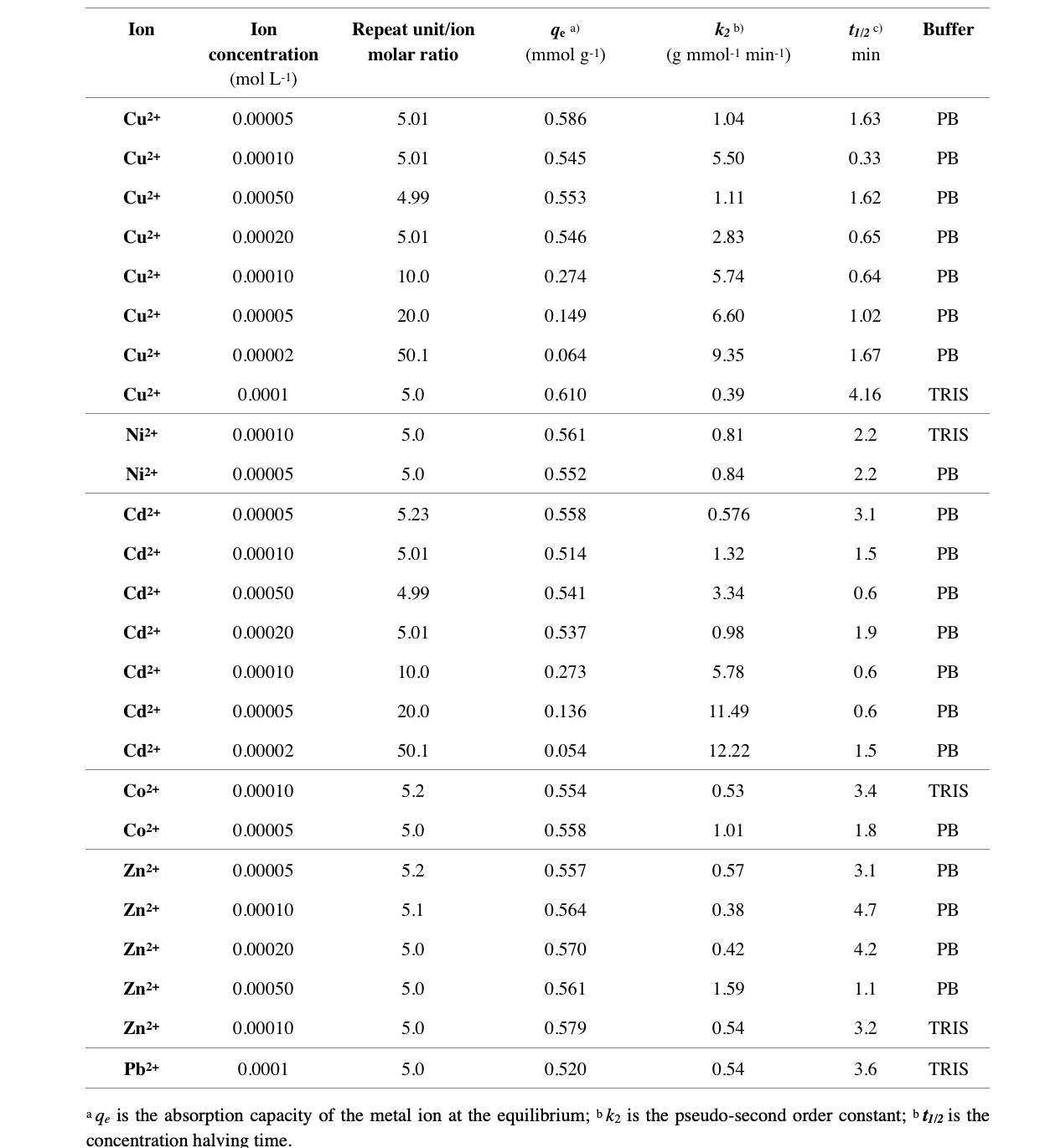
It may be observed that the apparent kinetic rate constant k2 regularly increased with increasing the resin repeat unit/ion metal ratio, being the resin weight the same, since the availability of coordinating sites increased correspondingly. The apparent rate constants k2 also increased with the metal concentration being the resin repeat unit/ion metal ratio constant. As regards the ability of LMT85 to make coordination complexes with the different ions considered, it is apparent that Ni2+ absorption was slower than Cu2+ absorption in phosphate buffer, but faster in TRIS buffer, possibly on account of some competition of TRIS in Cu2+ coordination. Cd2+ absorption rate was comparable to that of Cu2+, whereas significantly lower kinetic constants, hence higher halving times, were observed with Zn2+, Co2+ and Pb2+. In the case of Pb2+, the phosphate buffer was avoided to prevent precipitation.
Resin coloring
In a number of instances, ion absorption produced intense coloring, namely blue, pink/violet and green with particularly with Cu2+, Co2+ and Ni2+, respectively (Figure 4). This behavior may be also useful for analytical puroposes.
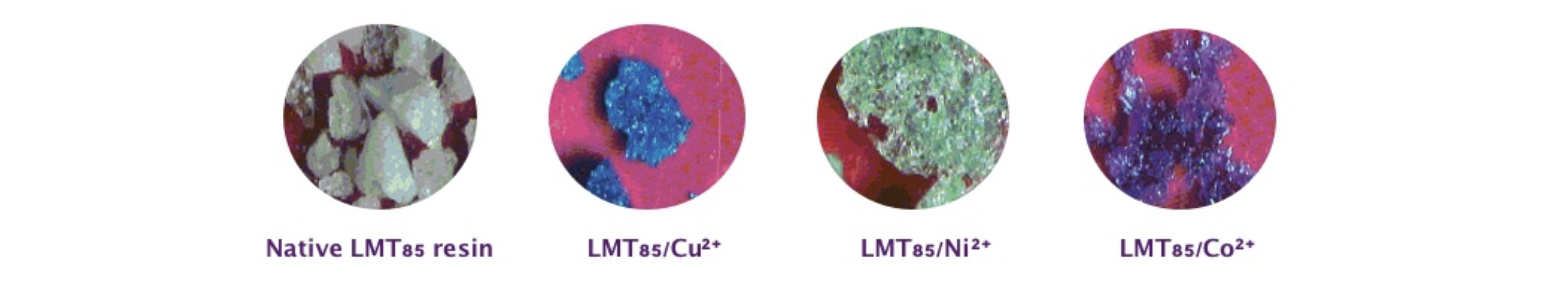
Figure 4. Coloring of LMT85 samples after absorption of heavy metal ions.
Conclusions
The EDTA mimic LMT85 resin proved capable of rapidly and quantitatively absorbing the six heavy metal ions considered, namely Cu2+, Ni2+, Co2+, Cd2+, Zn2+ and Pb2+ either singly or in mixed solution. The absorption rate was fast even from dilute solutions down at least to 0.00005 M (3 ppm). The resin was regenerated by acidifying with acid aqueous solutions. The absorption of several metal ions imparted intense colouring to the resins, a feature exploitable, in principle, for analytical purposes.
REFERENCES
[1] J. Briffa, E. Sinagra, R. Blundell “Heavy metal pollution in the environment and their toxicological effects on humans” Heliyon 2020, 6, e04691.
[2] P. Ferruti, E. Ranucci, A. Manfredi, N. Mauro, E. Ferrari, R. Bruni, F. Colombo, P. Mussini, M. Rossi “L-Lysine and EDTA polymer mimics as resins for the quantitative and reversible removal of heavy metal ion water pollutants” J. Polym. Sci. Part A: Pol. Chem. 2012, 50, 5000–5010.
[3] P. Ferruti. Polyamidoamines: past, present and future perspectives. J. Polym. Sci. Part A: Pol. Chem. 2013, 51, 2319–2353.
[4] DIN 38406 Teil 16, ‘‘Verfahren zur Bestimmung von Zink, Cadmium, Blei, Kupfer, Thallium, Nickel, Cobalt, mittels Voltammetrie’’, E16 Deutsche Einheitsverfahren.
[5] W. Joseph, Analytical Electrochemistry; Wiley-VCH, Hoboken, New Jersey, 2006.